About This Study
Females age 18 or older, diagnosed with primary invasive breast cancer and or ductal carcinoma in situ (DCIS) and scheduled for lumpectomy are eligible to participate. The INSITE study evaluates a novel drug (pegulicianine) and Lumicell’s imaging system for safety and efficacy in identifying and guiding removal of cancer remaining in the surgical cavity after the standard lumpectomy, with the goal of reducing the risk of repeat surgeries.
Pegulicianine is designed to fluoresce – or light up – in the presence of cancer cells to help surgeons differentiate between healthy and abnormal tissue. After your surgeon completes the standard lumpectomy procedure, your surgeon will scan the surgical cavity with Lumicell’s imaging system to identify and remove residual disease. The Lumicell technology has been used in multiple clinical studies in over 500 breast cancer patients.
My Treatment
Here is how your surgery and treatment plan will change if I you choose to participate in this study.
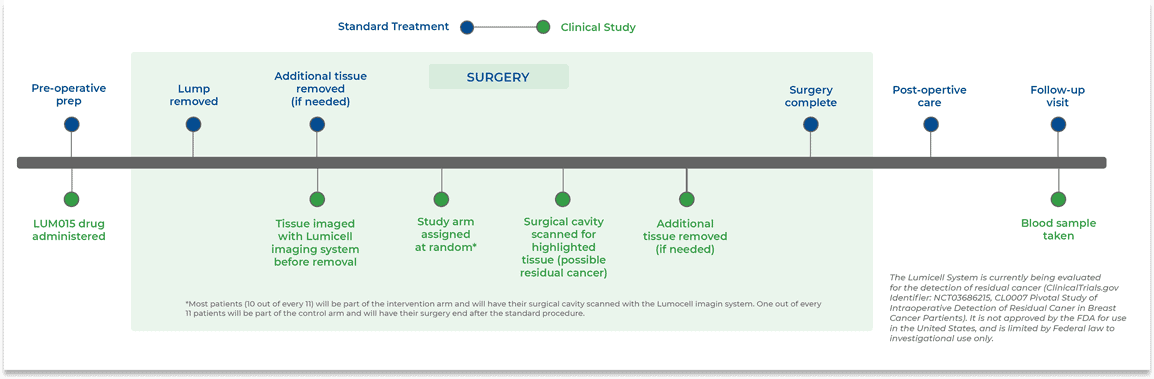
Commonly Asked Questions
How can I learn more?
Contact your doctor or enter your zip code below to find a doctor near you that is involved in the study.
Please wait...
| 1 | PENNSYLVANIA | 1 |
| 2 | FLORIDA | 3 |
| 3 | MASSACHUSETTS | 1 |
| 4 | NORTH CAROLINA | 2 |
| 5 | ALABAMA | 1 |
| 6 | OHIO | 1 |
| 7 | MICHIGAN | 1 |
| 8 | ARIZONA | 1 |
| 9 | CALIFORNIA | 1 |
| 10 | WASHINGTON | 1 |
| 11 | TEXAS | 1 |